dOFM Studies
Deeper Insights. Rapid Science-Based Decisions.
Fast, science-driven dOFM studies for early-phase drug interaction tests—providing continuous PK/PD data at the site of action and reliable results you can trust.
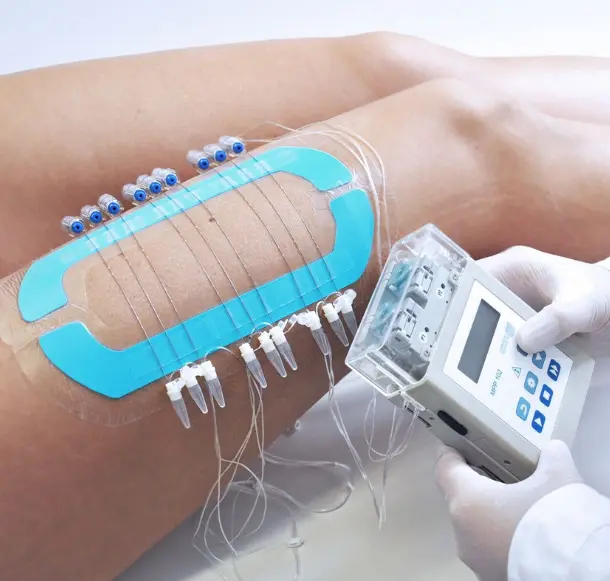
You Need dOFM Studies That Deliver Clarity, Without Delays
Traditional drug interaction studies can be slow, costly, and inconclusive, forcing sponsors to rely on guesswork before moving forward.
With dOFM studies at Axis, you can capture real-time, high-resolution PK/PD data directly from the skin, reducing uncertainty and accelerating smarter drug development decisions.
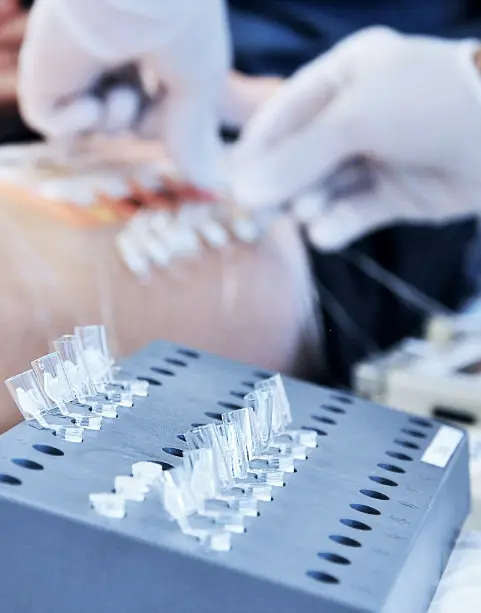
dOFM studies provide reliable insights for drug drug interaction studies, helping sponsors make faster, smarter choices.
Better Answers, Sooner
Axis utilizes Dermal Open Flow Microperfusion (dOFM) to give you earlier, richer PK/PD data from fewer subjects, so your program advances with speed and clarity.
- Smaller, Smarter Studies Obtain meaningful human data with as few as 8–10 volunteers, saving both time and cost.
- Deeper Insights dOFM continuously samples dermal interstitial fluid, revealing how your drug formulation truly behaves at its site of action.
- Better Development Decisions Earlier, richer data supports drug interaction studies, guiding dose selection, formulation changes, and regulatory strategies.
Rapid Enrollment & Seamless Execution
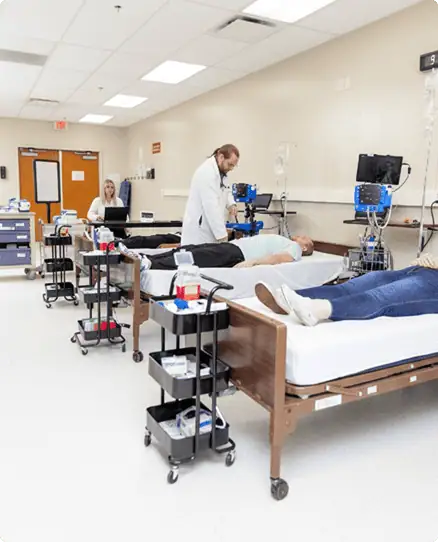
Our clinical pharmacology unit combines fast patient recruitment with dedicated infrastructure to keep your dOFM study moving on schedule.
Expansive Volunteer Database
Access to a broad pool of healthy subjects ensures faster enrollment.
Strategic Trial Location
Conveniently located near universities and diverse communities for strong participation.
Community Involvement
Local partnerships build trust and strengthen retention across studies.
Quick Start-up
Optimized processes help you begin your dOFM study within weeks, not months.
Scientific Precision Backed by Proven Experience
When you run dOFM studies, you need confidence that every data point reflects both laboratory rigor and in-human relevance. At Axis, your program is supported by:

Dermal Open Flow Microperfusion (dOFM) services delivering continuous PK/PD data for drug interaction studies, dose-response evaluation, and early proof of therapeutic effect.
- Expert Teams Clinical research specialists with deep experience in PK/PD profiling and dermal pharmacology.
- Regulatory-Ready Protocols Study designs aligned with global guidance for reproducibility and smooth submissions.
- Proven Track Record Successful execution of dOFM and related dermal pharmacology studies across multiple therapeutic areas.
- Tailored Study Designs Flexible approaches customized to your goals, timelines, and development strategy..


Axis is built to deliver TQT studies with unmatched efficiency, combining advanced technology with expert oversight to keep every trial moving forward.
Advanced Data Systems & Oversight You Can Trust
With dOFM, precision is everything. That’s why our systems and processes ensure your data is accurate, clean, and submission-ready.
Extended PK Profiling
dOFM captures detailed dermal pharmacokinetics for up to 72 hours, giving you deeper insight into drug behavior over time.
Direct Dose-Response Measurement
Measure dose-response directly in dermal tissues—no reliance on blood surrogates—providing a more accurate view of drug impact.
Early Proof of Effect
Demonstrate therapeutic effects and mechanisms of action in the dermis early, supporting faster, smarter development decisions.
High-Quality, Consistent Data with eSource and EDC System
ClinSpark® and integrated lab systems strengthen dOFM and DDI drug interaction studies with reliable real-time data.
Electronic Data Capture
Our ClinSpark® platform reduces errors and streamlines ADME and DDI study data by integrating with lab systems and medical devices.
Seamless Device Integration
Real-time data flows from ECGs, vitals, and lab systems.
Continuous Safety Monitoring
Bi-directional connectivity ensures oversight throughout your study.
Regulatory Alignment
Every dOFM study is conducted to meet FDA and global expectations for drug interaction guidance.
High-Quality Drug Interaction Studies Backed by Precision Systems
Full-Scope CRO Services
As a full-service clinical research organization, Axis delivers the end-to-end expertise you need to move your clinical trial forward.

Clinical research specialists delivering accurate, real-time results in dOFM and DDI drug studies.
- Clinical Development and Strategic Consulting
- Clinical Trial Management
- Project Management
- Support Services: Labs, IP Management
- linical Monitoring
- Medical Monitoring
- Data Management
- Biostats & Medical Writing
- QA & Regulatory Submissions
Accelerate Your dOFM Trial With Precision, Speed, and Trusted Data
Your Path to Fast, Confident dOFM Results
With dOFM studies, Axis helps you move quickly from early exploration to actionable results, combining innovative science with proven expertise to keep your trial advancing.

Schedule a
Consultation
Share your study needs,
goals, and timelines.

Get a Tailored
dOFM Proposal
Receive a transparent study plan aligned with your compound and development goals.
Advance Quickly
With Confidence
Gain early insights that strengthen your drug interaction studies and accelerate development.
Don’t Let Unclear Data Stall Your Program
Partner with a CRO built to deliver faster timelines, deeper insights, and reliable dOFM study results.

Free Guide:
5 Ways to Keep Your Phase I Trial on Schedule
A timely trial protects your milestones, budget, and momentum.
- Download our free guide and learn how to:
- Start faster without sacrificing quality
- Avoid common operational bottlenecks
- Keep your trial moving toward the next phase
Have questions or need expert support? Our team is here to help—reach out and let’s move forward together.