CARDIAC SAFETY STUDIES / TQT STUDIES
The Faster Pathway to Reliable Phase 1 Trial Results
High-quality cardiac safety studies andTQT studies, with the speed, precision, and efficiency you need to move forward with confidence.

You Need a CRO That Delivers Quality Cardiac Safety Data... Quickly
Cardiac safety and TQT studies should combine scientific rigor with proven speed to keep your clinical trial on track.
At Axis, we provide a dedicated clinical trial unit, rapid enrollment, and integrated cardiovascular monitoring to keep your study progressing while generating the reliable data regulators demand.

Clinical trial unit equipped for cardiac safety and TQT studies with real-time Holter and Telemetry data capture.
Comprehensive Cardiac Safety Services
From first-in-human through submission, our clinical research organization (CRO) delivers every major cardiac safety study design:
- Thorough QT (TQT) studies
- Cradiovascular monitoring / Telemetry
- Thorough QT (TQT) studies
- Integration with pharmacology, ADME, and dermatology programs
Rapid Recruitment for Cardiac Safety Trials
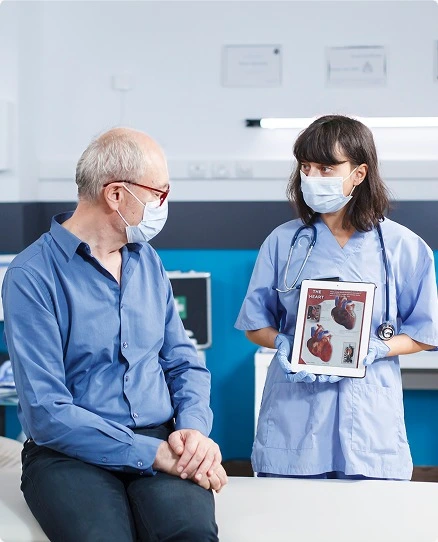
Cardiac safety studies are often time-sensitive. With one of the highest-enrolling clinical research centers in the U.S., AXIS ensures you meet enrollment targets quickly and keep your study moving.
Expansive Volunteer Database
A large, engaged pool of healthy volunteers ready for cardiovascular monitoring studies.
Strategic Location
Our Minnesota clinical trial unit draws from diverse populations and nearby universities.
Community Involvement
Strong ties with local communities boosts trust, participation, and retention.
Fast Start-up
First-patient-in within weeks, not months, with our optimized processes and quick enrollment.
Expertise in Cardiac Safety Studies
When you’re developing a new therapy, cardiac safety data is non-negotiable.
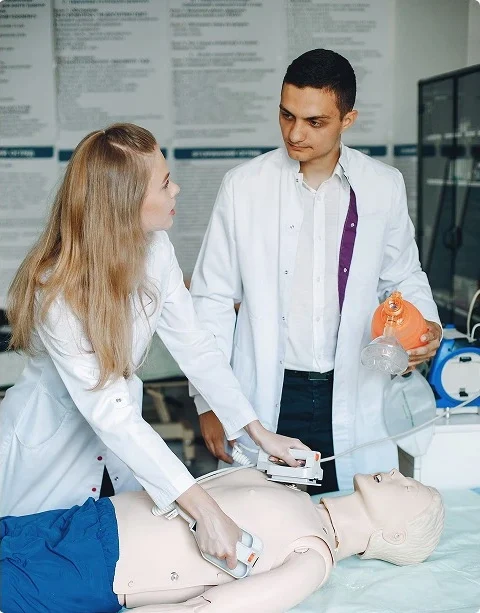
Cardiac safety and TQT studies led by experienced teams with regulatory-ready results.
Axis provides:
- High-Quality Cardiac Safety Data Accurate data ready for regulatory submissions.
- Proven TQT Studies Delivering reliable QT interval assessments that regulators expect.
- Advanced Cardiac Monitoring Trials Seamless Telemetry device integration ensures accuracy in cardiovascular monitoring.
- Experienced Clinical Research Specialists Oversight at every step to prevent costly errors or delays.
Our expert team—supported by dedicated clinical trial units, monitoring protocols, and hot labs for sample analysis—acts as an extension of your organization from Day 1.


Axis is built to deliver TQT studies with unmatched efficiency, combining advanced technology with expert oversight to keep every trial moving forward.
Technology + Expertise for Reliable Cardiac Safety Data
Your cardiac safety trial deserves uncompromising precision.
At AXIS, we combine technology, bioanalytical services, and expert staff to ensure accurate and timely results.
Real-Time Holter / Telemetry Capture
Seamless integration with Cardiac monitoring devices for error free data.
Continuous Safety Oversight
Real-time cardiovascular monitoring with bi-directional lab connectivity for total compliance.
Consistent Dosing Protocols
Pharmacists and trained staff ensure dosing accuracy across every subject.
Customized Project Execution
Tailored study plans keep timelines tight and stakeholders aligned.
High-Quality, Consistent Data with eSource and EDC System
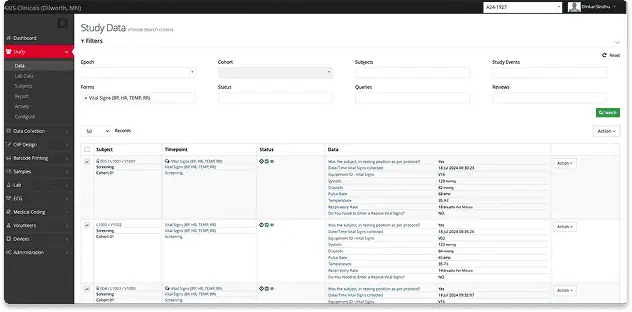
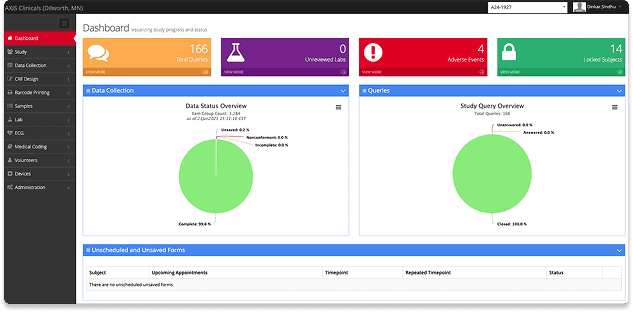
State-of-the-art clinical research center equipped for comprehensive cardiovascular monitoring and cardiac safety trials.
Electronic Data Capture
All study data is collected electronically in ClinSpark®, the industry-leading eSource/EDC platform built for Phase I trials.
Seamless Device Integration
Direct connections with ECGs, vitals monitors, and scales allow for real-time data capture while eliminating transcription errors.
Continuous Safety Oversight
Bi-directional connectivity with our clinical safety lab—using CDISC and HL7 standards—ensures ongoing, real-time safety monitoring throughout your study.
Precision Technology Driving Reliable Cardiac Safety Results
Full-Scope Clinical Research Organization
Axis offers end-to-end CRO services for cardiac safety and beyond:

End-to-end CRO services for cardiac safety, TQT studies, and early-stage clinical research programs.
- Clinical Development and Strategic Consulting
- Clinical Trial Management
- Project Management
- Support Services: Labs, IP Management
- Clinical Monitoring
- Medical Monitoring
- Data Management
- Biostats & Medical Writing
- QA & Regulatory Submissions
Keep your trial moving forward and on schedule with a CRO built for speed and precision.
Your Path to Fast, Reliable Cardiac Safety Data
Getting started doesn’t have to be complicated—our streamlined process makes it simple to launch your cardiac safety study and keep it on track.

Schedule a
Consultation
Share your study design, timelines, and endpoints with our experts.

Get a Custom Proposal
& Trial Timeline
Receive a clear, tailored roadmap with cost-efficient execution.
Advance Your Study
Quickly With Confidence
Hit cardiac safety milestones
on time with reliable, submission-ready data.
Don’t Let Missed Milestones Derail Your Cardiac Safety Study
Partner with a CRO in clinical research built to deliver fast, accurate cardiac safety and TQT studies.

Free Guide:
5 Ways to Keep Your Phase I Trial on Schedule
A timely trial protects your milestones, budget, and momentum.
- Download our free guide and learn how to:
- Start faster without sacrificing quality
- Avoid common operational bottlenecks
- Keep your trial moving toward the next phase
Have questions or need expert support? Our team is here to help—reach out and let’s move forward together.