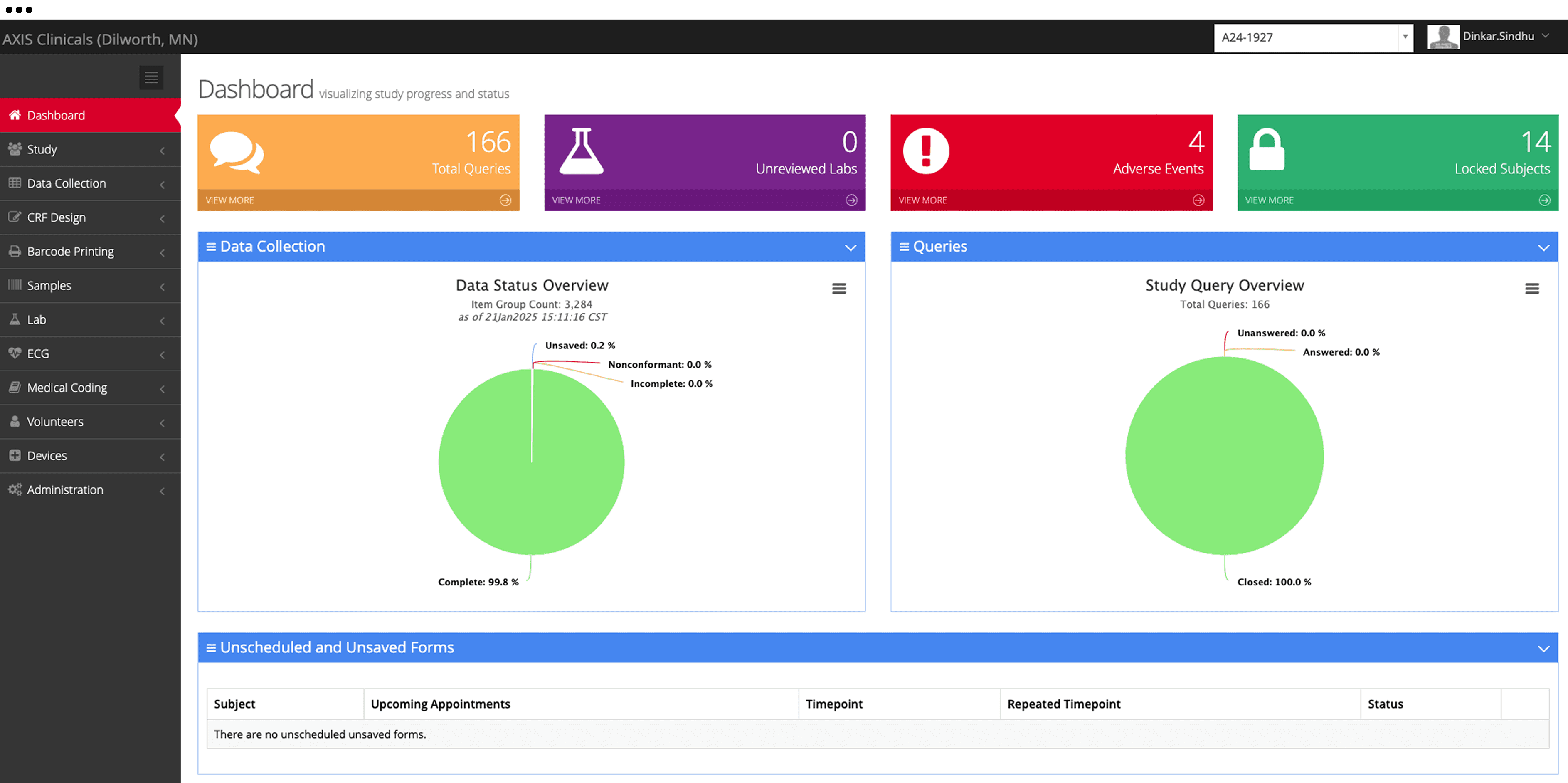
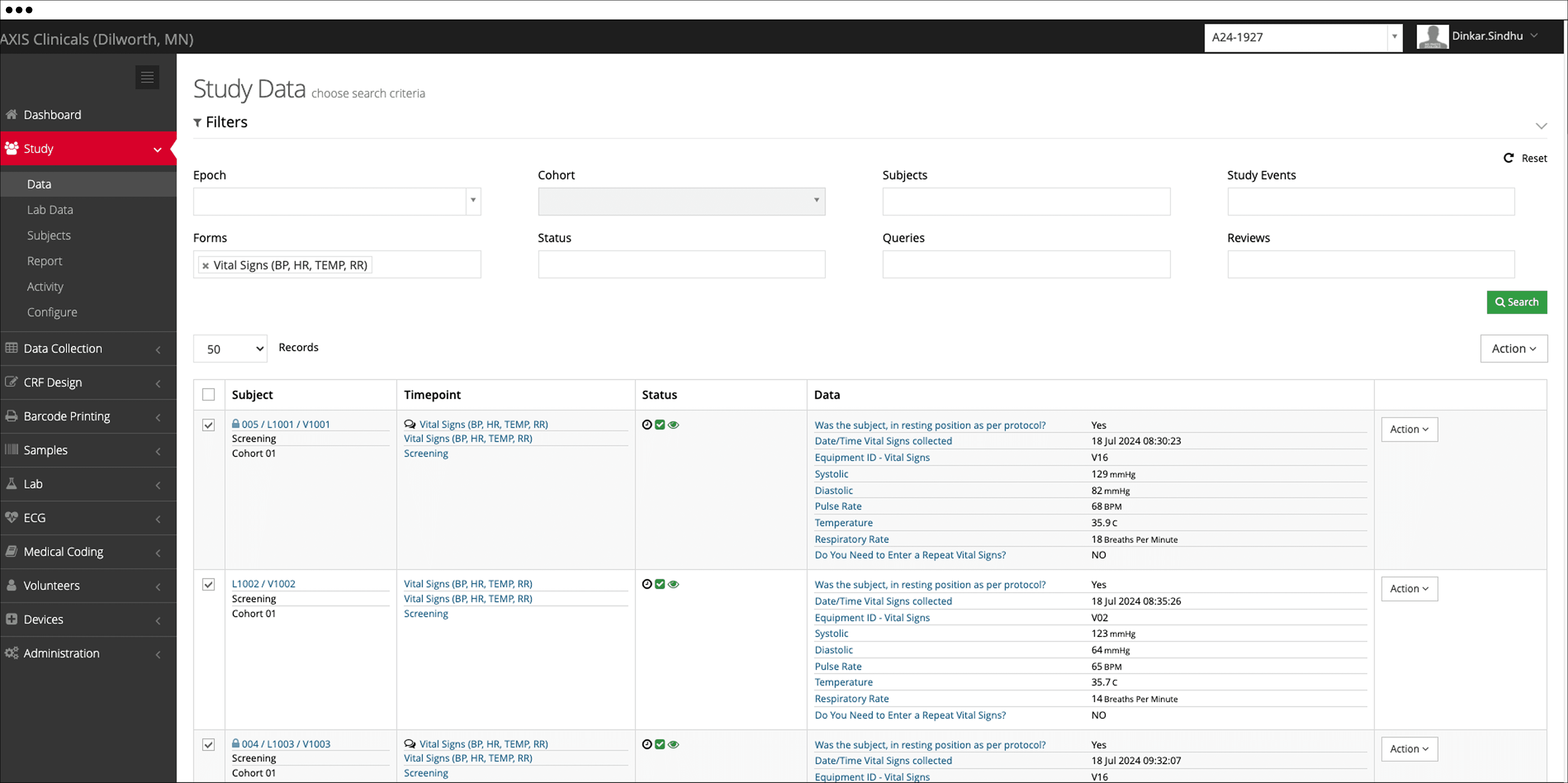
We are a leading clinical pharmacology CRO, well-equipped to accelerate the progression of your clinical development plan in clinical research.
Our onsite bioanalytical laboratory provides expedited analysis as part of our integrated clinical pharmacology services, especially in dose-escalation pharmacology studies.
Our recruitment and marketing team has streamlined the process for rapid recruitment, supporting our role as a specialized CRO in clinical research for pharmacology studies.
With our database of more than 20,000 volunteers at our Minnesota center, we have the capability to recruit for multiple studies every month.
Proximity to five universities and colleges provides us access to a large population of healthy volunteers.
We participate in many local community and university events to promote recruitment for your study.
Our pharmacists will aid you in the development of a robust drug preparation, compounding, and dosing protocol and provide training to ensure consistent dosing.
Our team’s highest priority is to achieve satisfaction of each of your stakeholders through project engagement to successfully execute the study.