hADME Studies
Understand Behavior. Accelerate Decisions.
Pharmacokinetics ADME studies done right-with fast insight, high scientific rigor, and regulatory-ready outputs.

You Need hADME Studies You Can Trust, That Don't Slow Your Program
Absorption, Distribution, Metabolism, Excretion (hADME) data is foundational for your IND, NDA, or global regulatory strategy. But delays can ripple through your development program.
At Axis, we deliver hADME services that are scientifically rigorous, yet optimized to move fast-so you can make important decisions on time.

ADME studies & metabolism profiling performed under tightly controlled conditions by expert pharmacokinetics ADME teams.
Complete hADME Study Capabilities
From early screening to full in-human metabolism assessments, our ADME study services span the full range:
- In vitro ADME screening (metabolic stability, enzyme phenotyping)
- Transporter studies and drug-drug interaction (DDI) assessments
- Human ADME studies to quantify absorption & excretion in real volunteers
- Pharmacokinetics ADME profiling to support dose selection
- ADME metabolism pathways clarification for regulatory filings
Rapid Enrollment & Sample Turnaround

You don’t want bottlenecks. With our high-enrolling volunteer cohort, well-equipped labs, and streamlined sample workflows, Axis gets you from sample collection to actionable ADME data quickly.
Large Volunteer Database
Healthy volunteers ready for human ADME studies.
Integrated Bioanalytical Lab Services
On-site labs with LC-MS/MS systems accelerate metabolite analysis.
Efficient In Vitro ADME Services
Fast screening of metabolic stability and enzyme interactions.
Quick Start-Up
First sample batch analysis begins quickly once your study is initiated.
Deep ADME Expertise & Scientific Rigor
When you run ADME studies, you need both lab precision and in-human relevance. At Axis, your ADME study is backed by scientific expertise and proven processes.

Bioanalytical laboratory services focused on ADME screening, in vitro ADME metabolism, and pharmacokinetics ADME profiling.
- Experienced clinical research specialists in pharmacokinetics ADME and metabolism assessment.
- Regulatory-Ready Protocols built for reproducibility and submission readiness.
- Strong track record with both in vitro ADME studies and full human ADME study designs.
- Customized clinical trials tailored to your needs and timeline.


Axis combines a dedicated clinical trial unit with an onsite hot lab, streamlining hADME studies for faster sample handling, greater accuracy, and seamless pharmacokinetics ADME data flow.
Advanced Data Systems & Quality Oversight
Quality is non-negotiable when regulatory agencies assess your ADME data. We use advanced systems and expert oversight to ensure your data is clean, accurate, and defensible.
Electronic Data Capture
Full lab integration with electronic data capture reduces transcription errors and improves data integrity.
Rigorous Compliance
Robust QC/QA processes throughout sample analysis and
pharmacokinetic modeling safeguard regulatory acceptance.
Trusted Analytics
Biostatistics and pharmacokinetics analysis are performed to meet FDA and global compliance standards.
Real-Time Monitoring
Continuous connectivity between lab systems and clinical operations provides instant oversight of safety and performance.
High-Quality, Consistent Data with eSource and EDC System
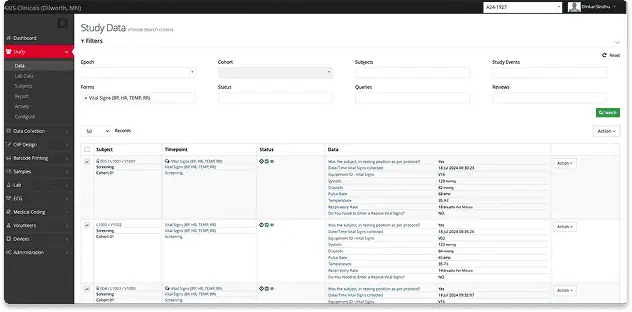
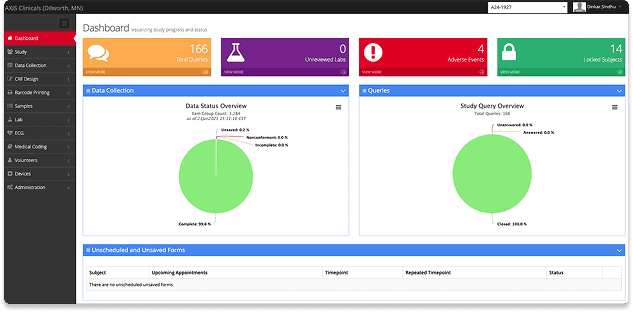
High-quality ADME study data through electronic data capture and seamless lab systems.
Electronic Data Capture
Every data point is collected electronically in ClinSpark®, the leading eSource/EDC platform for Phase I trials, ensuring accuracy from the start.
Seamless Device Integration
Direct connections with ECGs, vitals monitors, and scales allow for real-time data capture while eliminating transcription errors.
Continuous Safety Oversight
Bi-directional connectivity with our clinical safety lab-using CDISC and HL7 standards-provides real-time oversight to safeguard subjects and strengthen regulatory confidence.
Accurate ADME Studies Powered by Proven Technology
All-In One ADME CRO Support
Axis offers end-to-end CRO services for ADME studies

End-to-end CRO services for ADME studies and early-stage clinical research programs.
- Clinical Development and Strategic Consulting
- Clinical Trial Management
- Project Management
- Support Services: Labs, IP Management
- Clinical Monitoring
- Medical Monitoring
- Data Management
- Biostats & Medical Writing
- QA & Regulatory Submissions
Keep your for ADME study moving forward and on budget with a CRO built for speed and accuracy.
Your Path to Early-Stage ADME Study Confidence
Getting clear ADME study data doesn’t need to slow down or drag out your program. Here’s how we help you get there smoothly.

Schedule a
Consultation
Share your compound,
metabolism questions, and timelines.

Get a Custom Proposal
& Trial Timeline
Receive a transparent plan with scope, assay details, and schedule.
Gain Clarity
& MoveForward Fast
Gain early insights that strengthen your drug interaction studies and accelerate development.
Don't Let Unclear ADME Data Delay Your Progress
Choose a CRO built for speed, precision, and actionable ADME studies.

Free Guide:
5 Ways to Keep Your Phase I Trial on Schedule
A timely trial protects your milestones, budget, and momentum.
- Download our free guide and learn how to:
- Start faster without sacrificing quality
- Avoid common operational bottlenecks
- Keep your trial moving toward the next phase
Have questions or need expert support? Our team is here to help—reach out and let’s move forward together.