AXIS is supporting clinical research for NDA, 505(b)(2)
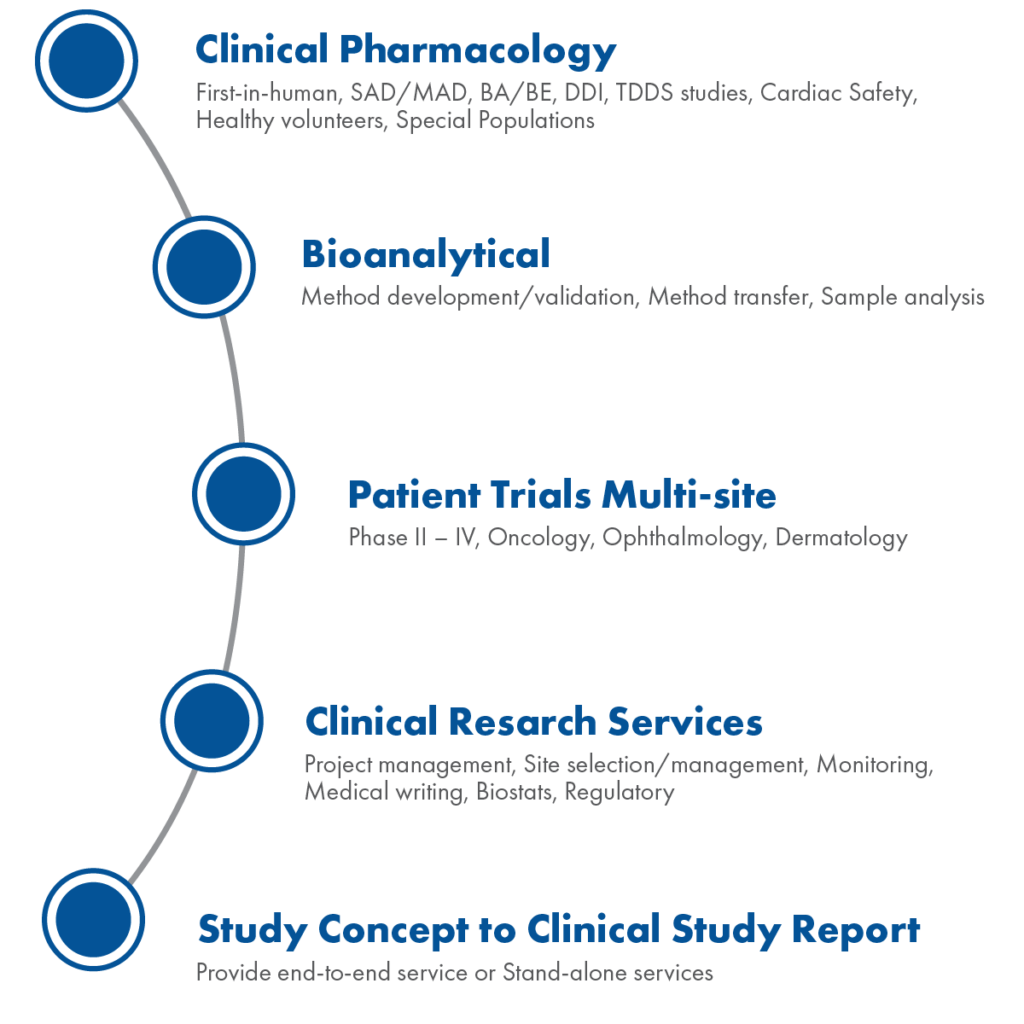
- Pharmacology Enabling Clinical Projects
- Ophthalmology, Oncology, Dermatology & Others
- Encompassing full-service CRO support
Phase 1 Early Stage Capabilities:
- First in Human/Proof-of-concept
- Pharmacokinetic/Pharmacodynamics
- Single Ascending Dose (SAD)
- Multiple Ascending Dose (MAD)
- Drug-Drug Interaction (DDI)
- Food Effect (FE)
- Gender and Age Effect
- Renal Impaired Studies
- Hepatic Impaired Studies
- Cardiac Safety Studies
US Patient Trials Therapeutic Areas:
Ophthalmology
Glaucoma, Cataracts, Contact Lens, Cornea, Refractive Indications, Retinal conditions, POC, AMRD, RP, Uveitis
Oncology
Breast cancer, Solid tumors, Hematological malignancies, PTCL
Dermatology
Acne, Actinic keratosis, Age spots, Atopic dermatitis, Bullous pemphigoid, Canker store, Chronic hives, Contact dermatitis, Cutaneous lupus, Dermatitis, Dermatomyositis, Genetic skin disorders, Graft-versus-host disease, Hair loss, Hemangioma, Hyperhidrosis, Keloid scar, Moles, Psoriasis, Rosacea, Spider veins, Sun allergy, Vasculitis, Vitiligo, Wrinkles
Our Therapeutics Experts:

Dr. Sai Chavala (Ophthalmology)
Dr. Chavala is a clinician-surgeon-scientist, dedicated to innovation and development of novel treatments that improve the quality of life of patients. After obtaining his MD, he completed residency/fellowship at Weill Medical College of Cornell University and Duke University. His research has been funded by NIH. He is a Professor of Surgery at TCU/UNTHSC School of Medicine in Fort Worth.
Indications:
Glaucoma, Cataracts, Contact Lens, Cornea, Refractive Indications, POC, ARMD, RP, Uveitis

Dr. Michael J. Blankinship (Dermatology)
Board-certified dermatologist, Dr. Blankinship provides comprehensive care to patients with various types of skin conditions and diseases. He lives in Fargo with his wife, Sunny and their three children. He and his family enjoy traveling and spending time at their lake cabin. He is originally from the Upper Peninsula of Michigan and enjoys hunting and fishing. He was a Big Ten bass fishing champion in 1999.
Indications:
Acne, Actinic keratosis, Age spots, Atopic dermatitis, Bullous pemphigoid, Canker sore, Chronic hives, Contact dermatitis, Cutaneous lupus, Dermatitis, Dermatomyositis, Genetic skin disorders, Graft-versus-host disease, Hair loss, Hemangioma, Hyperhidrosis, Keloid scar, Moles, Psoriasis, Rosacea, Spider veins, Sun allergy, Vasculitis, Vitiligo, Wrinkles

Dr. Erard M. Gilles (Oncology)
20 years’ experience in the pharmaceutical industry covering: R&D experience in large and small companies, creation and development of Invivis Pharmaceuticals Inc., Medical Affairs and Marketing
Indications:
Breast Cancer, Solid Tumors, Hematological Malignancies, PTCL
For further information, please feel free to contact John Pottier, Senior Vice President Business Development 913-314-6755 j.pottier@axisclinicals.com

